A Clinical Study for People with Cystic Fibrosis (CF)
Who Have Specific Mutations in the CFTR Gene, Which Are the Cause of CF
This clinical study is exploring a potential new inhaled messenger RNA
(mRNA) treatment called RCT2100 that may improve the function of
the CFTR protein in the lungs and can lead to protein production,
getting at the root cause of CF lung disease rather than just controlling
symptoms.
Here, you will find more information about the study, investigational medicine, and how you, or someone you know, can participate.
About Cystic Fibrosis
CF is a progressive genetic disease that causes persistent lung infections and respiratory failure. It is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and impacts approximately 130,000 people worldwide.
The absence or dysfunction of the CFTR protein results in a defect in airway hydration, which leads to excessive mucus buildup in the lungs. It also creates a mucociliary clearance defect, recurrent infections, inflammation, respiratory failure, and other complications.
Despite the availability of CFTR modulators that work for 90% of people with CF, many will not be able to take the medication due to side effects, lack of response, or other reasons. About 10% of the CF community have genetic mutations that do not benefit from these therapeutics.
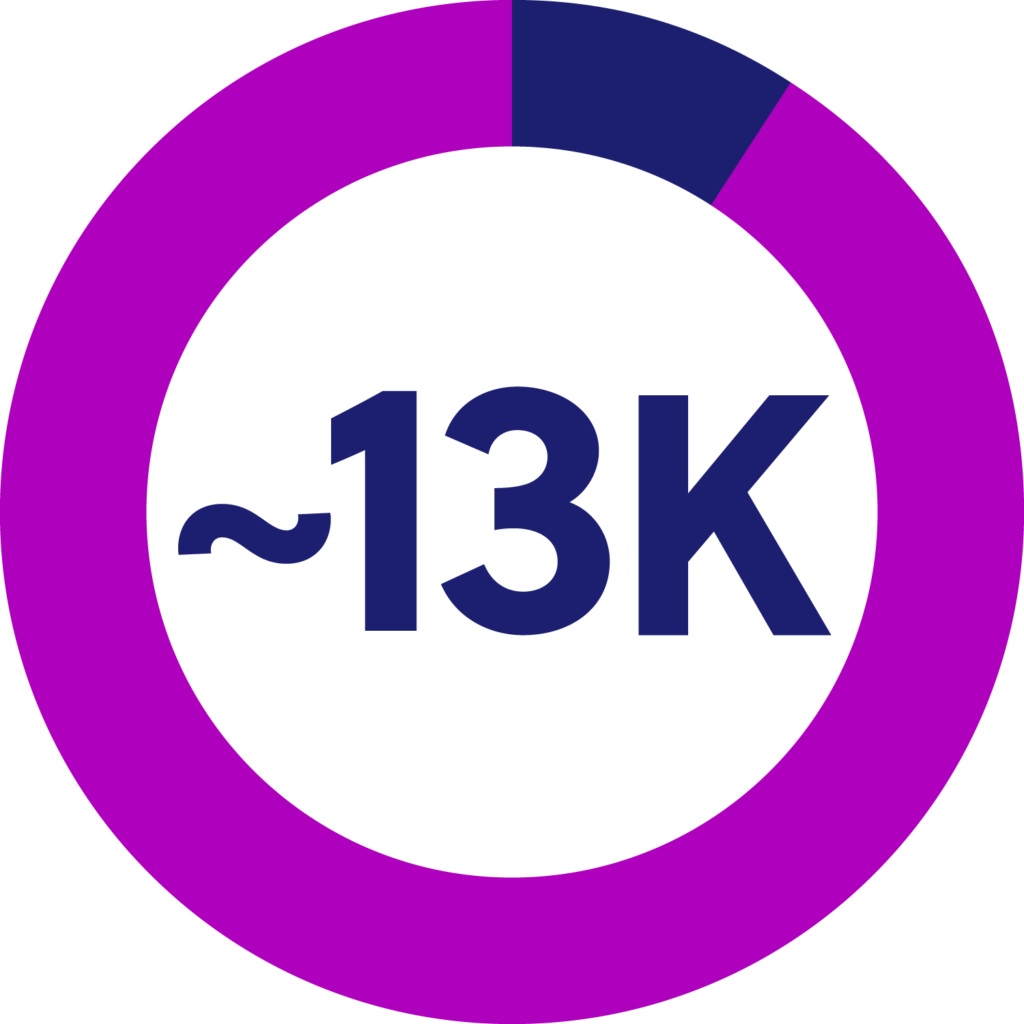
CF impacts approximately 130,000 people worldwide. 90% have responsive genotypes to CFTR modulators, and 10% (13,000 people) do not.
Why this Study is Important
No CFTR modulator therapies are currently approved to treat individuals with certain mutations in the CFTR gene because these mutations prevent the production of any CFTR protein.
Without CFTR protein, there is nothing available for the therapy to correct. This study aims to find out whether the investigational study medicine is safe and may work as a potential new treatment option for people with CF who are not eligible for, or are eligible but not currently taking, CFTR modulator therapy. The study is also testing to see if taking the investigational study medicine, RCT2100, with the already approved CFTR modulator therapy, ivacaftor, is safe and may provide greater benefit to people with CF who are not eligible for CFTR modulator therapy or who are eligible to receive ivacaftor in combination with other CFTR protein correctors.
To learn more, contact:
How the Clinical Study Medicine Works
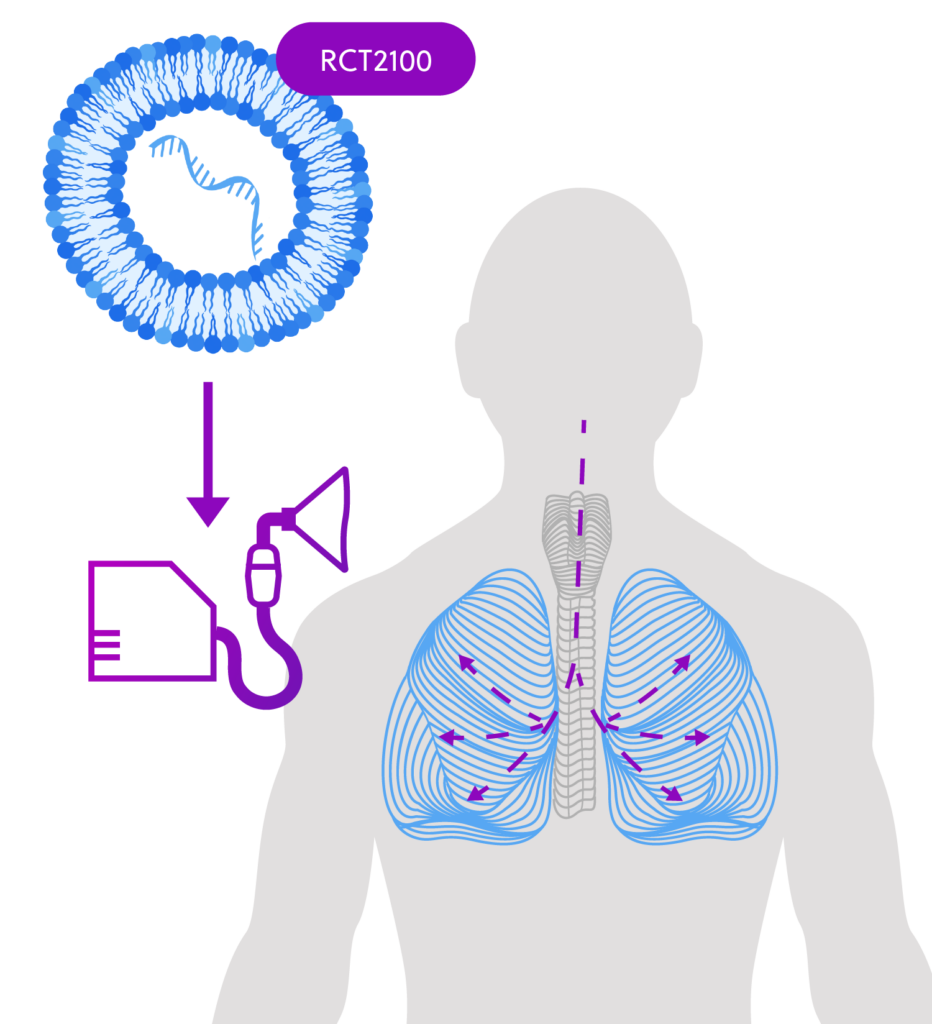
ReCode is developing RCT2100, an investigational inhaled therapy for cystic fibrosis (CF) based on messenger RNA (mRNA). This treatment is designed to deliver CFTR mRNA directly to target cells in the lungs, instructing them to produce a functional version of the CFTR protein.
Unlike current CFTR modulators that work on existing defective proteins by either correcting or potentiating that protein, RCT2100 is designed to allow the cells to make new functional CFTR protein.
By providing CFTR mRNA to individuals with CF who are missing this protein, the therapy aims to address the underlying cause of the disease, potentially improving CFTR protein function in the lungs rather than just managing symptoms.
RCT2100 administered with ivacaftor, a CFTR modulator that potentiates existing CFTR protein, may provide more improvement in CFTR protein function in the lungs than RCT2100 alone.
How the Clinical Study Medicine is Given
The investigational medicine, RCT2100, will be given using a nebulizer — a medical device that converts liquid medication into a mist for inhalation.
1 Screening
There will be a 2–4-hour office visit to determine your eligibility to participate and an opportunity for you to ask any questions to see if this study is right for you.
2 Treatment
If you are eligible, you will begin treatment with ivacaftor alone (taken orally) for 2 weeks followed by inhaled doses of RCT2100 taken in combination with ivacaftor (taken orally) for 4 weeks.
3 Follow-up
After your last dose, there will be a 24-week follow-up period, which includes a final outpatient visit.
Study tests and procedures
Throughout the study, you will have certain exams and tests, including:
Physical Exam
Vital Signs Measurement
A non-invasive test to record the heart’s electrical activity
Chest X-Ray
(if you have not had one within six months)
A non-invasive test that measures oxygen in the blood
Spirometry
A non-invasive test to measure how much and how fast you can move air into and out of your lungs and how well your lungs are exchanging gases
Blood and Urine Tests
Eligibility
- Those who are 18 to 60 years old
- Those with a diagnosis of CF
Are not eligible for treatment with CFTR modulators based on having mutations of the CFTR gene or eligible for CFTR modulators (except for ivacaftor), but not taking them
- Anyone with a forced expiratory volume (FEV1) between 50% and 100% (inclusive) predicted
- Those willing to use contraception
- Those who are pregnant or breastfeeding
- Anyone who has received treatment with a CFTR modulator in the last 12 weeks
- Those who have had a CF pulmonary exacerbation in the last 4 weeks
- Anyone with a lung infection caused by organisms including B. cenocepacia, B. dolosa or M. abscessus
- An oxygen saturation <94% on room air
FAQs
What is a clinical research study?
A clinical research study is a carefully designed investigation to assess the safety, effectiveness, and potential benefits of medical treatments, interventions, or procedures in humans. These studies are essential for advancing medical knowledge and improving patient care.
Participants in a clinical study may receive new investigational treatments and are closely monitored to observe their responses and outcomes. The studies follow strict ethical guidelines and regulatory requirements to ensure the safety and rights of participants.
By participating in a clinical research study, people with CF contribute to developing new medical therapies and may gain access to innovative treatments that might not yet be widely available.
Where will this study take place?
Study sites will enroll in the U.S. and U.K., as well as in France and the Netherlands. Click here to find a list of all available study sites.
How much of my time will this clinical research study take up?
If you are eligible and decide to participate in the study, the research team will
share more information about the time expected from you during the study.
There are three periods to the study:
- Screening period: 2-4-hour office visit to determine your eligibility to participate
- Treatment period: 6 weeks: 2-weeks with ivacaftor alone followed by 4 weeks of administration of RCT2100 and ivacaftor, Note: In some countries, there is a possibility to explore home health options for some part of this treatment period, depending on the site
- Follow-up period: 24 weeks
Will I receive compensation for participating in the study?
Will I be responsible for the cost of travel and other items to participate?
What are the risks of participating in a clinical research study?
Participating may involve some potential risks, which may occur from the study drugs or the tests.
You will receive an informed consent form describing the full risks involved before agreeing to participate in the study.
The research team will also discuss risks with you and answer any questions that you may have about the study.
What are the benefits of participating in a clinical research study?
Participating in a clinical research study for CF may contribute to the advancement of medical science, helping to develop new treatments for CF that can benefit others in the future.
It is difficult for me to travel to the study site for dosing up to three times a week. Can I still participate?
With the research team’s approval, this trial offers a home visit option. This option allows for dosing at your home for certain study visits, carried out by a research nurse trained to administer RCT2100. The research nurse will arrive one hour before dosing and remain at your home one-hour post-dose.
Can I change my mind regarding participation?
The withdrawal process will be discussed in detail at the initial consultation.








